Category: Prescription Drug
Choose eMedical online pharmacy Australia for all your prescription, non prescription and compounding pharmacy requirements.
What is in this leaflet
This leaflet answers some common questions about Oralair Initiation Treatment and Oralair Continuation Treatment sublingual tablets.
It does not contain all the available information.
It does not take the place of talking to your doctor.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking Oralair against the expected benefits it will have for you.
If you have any concerns about taking this medicine, ask your doctor.
What is Oralair used for
Oralair contains an allergen extract. Treatment with Oralair is intended to increase the immunological tolerance towards certain grass pollens, thereby reducing the allergic symptoms.
Oralair is used for the treatment of certain grass pollen allergies that are characterised by rhinitis (sneezing, runny or itchy nose, nasal congestion) with or without conjunctivitis (itchy and watery eyes) in adults, adolescents and children above the age of 5.
Before treatment, the allergy is confirmed by a doctor with adequate training and experience in allergic diseases, who will perform the appropriate skin and/or blood tests.
The grass pollens that Oralair is used for are: Dactylis glomerata (cocksfoot); Lolium perenne (rye grass); Anthoxanthum odoratum (sweet vernal grass); Phleum pratense (timothy) and Poa pratensis (meadow grass).
How it works:
Treatment with Oralair is intended to increase the immunological tolerance towards grass pollens, thereby building immunity to the specific allergens in grass pollens, thus helping to reduce the allergic symptoms.
Ask your doctor if you have any questions why Oralair has been prescribed for you.
Oralair is not addictive.
This medicine is available only with a doctor’s prescription.
Before you are given Oralair
Do not take Oralair:
- if you are hypersensitive (allergic) to any of the other ingredients of Oralair;
- if you suffer from severe and/or unstable asthma;
- if your immune system is very weakened or if you suffer from a disease that attacks your own immune system;
- if you suffer from a malignant disease (for example cancer);
- if you have any inflammation in your mouth (for example mouth ulcers, bleeding gums).
If you are not sure if you should be taking Oralair, talk to your doctor.
Before you start your treatment with Oralair:
Take special care with Oralair:
If you have to undergo surgery in the mouth or if you are having a tooth pulled, you should stop treatment with Oralair for 7 days to allow your oral cavity to heal. Thereafter, restart treatment with the previous dosage. If you stopped treatment for more than 7 days, please ask your doctor how you should restart treatment.
Talk to your doctor if :
- you have any history of allergic inflammation of the oesophagus (eosinophilic oesophagitis). During treatment with Oralair, if you have severe or persistent upper abdominal pain, swallowing difficulties or chest pain, please contact your doctor who may reconsider your treatment.
- you are taking a beta blocker (i.e., a class of drugs often prescribed for heart conditions and high blood pressure but also present in some eye drops and ointments), as this drug may decrease the effectiveness of adrenaline, a medicine used to treat serious allergic reactions.
Taking other medicines
Tell your doctor if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Pregnancy and breast-feeding
Ask your doctor for advice before taking any medicine.
At present there is no experience for the use of Oralair during pregnancy. Therefore, you should not start immunotherapy if you are pregnant. If you become pregnant, speak to your doctor about whether it is appropriate for you to continue treatment.
There is no experience for the use of Oralair during breast-feeding as well. No effects on infants who are breast-fed during treatment are anticipated. However, you should not start immunotherapy if you are breast-feeding. If you wish to breastfeed while on treatment, speak to your doctor about whether it is appropriate for you to continue treatment.
Driving and using machines
No effect on the capacity to drive or use machines has been observed with Oralair.
Important information about some of the ingredients of Oralair
This medicine contains lactose. If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicinal product.
Your doctor will advise you.
Your doctor has more information on medicines to be careful with or avoid while taking Oralair.
How Oralair is given
Treatment should be started 4 months before the onset of the pollen season.
Always take Oralair exactly as your doctor has told you. Check with your doctor if you are not sure.
Oralair is prescribed by doctors with adequate training and experience in treatment of allergy. With prescriptions for children, the doctor will have the relevant experience in the treatment of children.
You are advised to take the first tablet under medical supervision and be monitored for 30 minutes. This gives you the opportunity to discuss possible side effects with your doctor.
Dosage
Adults, adolescents and children above the age of 5
The therapy is composed of an initiation treatment (first month of treatment, including a 3-day dose escalation) and a continuation treatment.
Initiation Treatment:
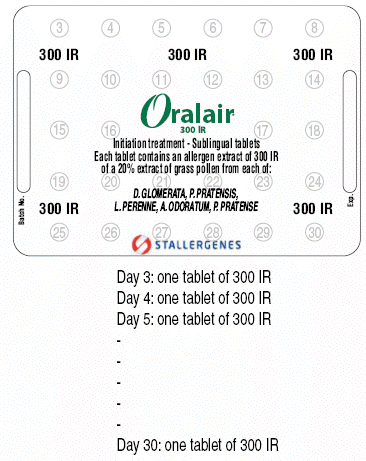
The pack corresponding to the initiation treatment (first month of treatment) contains two blisters:
- One small blister with 3 tablets of 100 IR
- One large blister with 28 tablets of 300 IR
Small blister
Method of administration
Take one 100 IR tablet on day one. Keep the tablet under your tongue until it is completely dissolved (for at least 2 minutes) before you swallow. On the second day, two 100 IR tablets are put under the tongue simultaneously and then swallowed after about 2 minutes. It is recommended that the tablets be taken during the day.
Use the following dosing scheme:
Always start with the small blister:

Large blister
Continuation Treatment:
The package corresponding to the continuation treatment (from the second treatment month) contains larger blisters packs with 30, 90, or 100 tablets of 300IR sublingual tablets
From the 2nd month onwards, treatment must be continued until the end of the pollen season with 1 tablet of 300 IR once a day from the Oralair CONTINUATION TREATMENT which contains 300 IR sublingual tablets only.
Duration of treatment
Take these tablets as prescribed by your doctor. Start treatment about 4 months before the beginning of the pollen season and continue it until the end of the pollen season.
Treatment is to be repeated over 3 consecutive grass pollen seasons.
If necessary, your doctor may, at the same time, prescribe medication to treat the possible allergic symptoms.
There is no experience with Oralair in young children (younger than 5 years) and in patients over 65 years of age.
How long should Oralair be taken for?
Oralair is started 4 months before the pollen season and then continued until the pollen season ends. Each year your Doctor will assess if it is suitable for you to restart Oralair treatment. Results from a recent long term study have shown that patients treated over 3 consecutive pollen seasons with Oralair, did not require further treatment in the 4th year.
If you take more Oralair than you should
If you take more Oralair than you should, you may experience allergic symptoms including local symptoms from mouth and throat. If you experience severe symptoms, such as angioedema (i.e. swelling), difficulty in swallowing, difficulty in breathing, changes in voice, or a feeling of fullness in the throat, seek urgent medical advice.
Contact the Poisons Information Centre on (13 11 26 in Australia and 0800 POISON (0800 764 766) in New Zealand) for advice on the management of overdose.
If you forget to take Oralair
Do not take a double dose to make up for a forgotten dose.
If you interrupted treatment with Oralair for less than one week, you can take up treatment where you left off.
If you stop taking Oralair
If you stop taking Oralair before the full treatment end, you may not have an effect from the treatment.
If you have any further questions on the use of this product, ask your doctor.
While you are receiving Oralair
Things you must do
Tell all doctors, dentists and pharmacists who are treating you, that you are receiving Oralair.
Tell your doctor if you feel the treatment is not helping your condition.
Side effects
Oralair helps most people with allergies to the selected group of grass pollens but they may have unwanted side effects in a few people.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of these side effects.
Contact your doctor or nurse immediately if symptoms of an allergic reaction occur.
During treatment with Oralair, you will be exposed to substances that may cause application site reactions and/or symptoms, which affect the whole body. You may experience application site reactions (such as itching of the mouth and throat irritation). These reactions usually occur at the beginning of therapy and are temporary, generally diminishing over time.
Stop taking ORALAIR and contact your doctor immediately if you develop or notice:
Allergic symptoms that affect the whole body (i.e. rapid onset of an illness associated with involvement of the skin and/or mucosa, breathing difficulty, abdominal pain or symptoms related to a drop in blood pressure).
Other possible side effects include the following:
Very common (affects more than 1 in 10 people)
- Throat irritation, itchy mouth, headache.
Common (affects less than 1 in 10 people):
- Asthma
- Stomach pain, diarrhea, vomiting
- Rhinitis (stuffy nose, runny nose, sneezing, itchy nose, nasal discomfort)
- Inflammation in the eyes, itchy eyes, watery eyes
- Itchy ears
- Swelling or itching of lips
- Swelling or itching or pain of the tongue
- Mouth disorders (such as dryness, tingling, numbness, inflammation, pain, blistering or swelling)
- Throat disorders (such as dryness, discomfort, pain, blistering or swelling)
- Difficulty in swallowing
- Hoarseness
- Cough
- Chest discomfort
- Heartburn, upset stomach
- Nausea
- Itching
- Hives
- Difficulty in breathing
- Congestion of sinus
- Persistent skin condition characterized by dryness
- Redness and itching
- Skin lesions subsequent to scratching
- Inflammation of the mouth
- Nose and throat inflammation
Uncommon (affects less than 1 in 100 people):
- Dry eye, eye redness, swelling around the eyes
- Ear discomfort, ear infection
- Inflammation of the gums or lips or tongue
- Tongue ulceration
- Swollen palate
- Salivary gland enlargement, overproduction of saliva
- Throat numbness, throat tightness, foreign body sensation in the throat
- Allergic reaction with swelling of the face and throat
- Belching
- Swollen lymph nodes
- Rash
- Acne
- Cold sores
- Flu-like illness
- Altered taste
- Sleepiness
- Dizziness
- Depression
- Hypersensitivity
- Sneezing
- Tiredness
- Mouth ulceration
Rare (affects less than 1 in 1000 people):
- Flushing
- Facial swelling
- Increase of eosinophil count (related to immune system blood cells)
- Anxiety
Side effects in children and adolescents
The following adverse reactions were more frequent in children and adolescents than in adults who received ORALAIR
Very common (affects more than 1 in 10 people)
- Cough
- Nose and throat inflammation
- Mouth oedema
Common (affects less than 1 in 10 people)
- Oral allergy syndrome
- Lip inflammation
- Lump feeling in the throat
- Tongue inflammation
- Ear discomfort
In children and adolescents the following additional adverse reactions were also reported:
Common (affects less than 1in 10 people)
- Bronchitis
- Tonsillitis
Uncommon (affects less than 1 in 100 people):
- Chest pain
Additional side effects experienced in actual use (post marketing experience, frequency unknown)
- Worsening of asthma
- Systemic allergic reaction
- Esophageal inflammation
These do not represent all possible adverse drug reactions. For a full list of all reported adverse drug reactions refer to the Product Information or your Doctor.
After using Oralair
Storage
- Keep it where children cannot reach it
- Keep it in the original pack
- Keep it below 30ºC.
- Do not use if past the expiry date which is stamped on the carton side panel
Product Description
Oralair Initiation Treatment sublingual tablets 100 IR and 300 IR contain a small blister with 3 tablets of 100 IR concentration and a large blister with 28 tablets of 300 IR concentration.
OR
Oralair Initiation Treatment sublingual tablets 100 IR and 300 IR contain a small blister with 3 tablets of 100 IR concentration and a large blister with 7 tablets of 300 IR concentration.
Oralair Continuation Treatment sublingual tablets 300 IR contain blisters with 30 tablets of 300 IR concentration. The continuation treatment is available in packs of 30, 90 or 100 tablets.
The tablets are round, biconvex, white to beige, and slightly speckled. The 100 IR tablet is engraved with 100 on both sides while the 300 IR tablet is engraved with 300 on both sides.
Presentation
The following pack sizes are available:
Oralair Initiation Treatment sublingual tablets 100 IR and 300 IR (AUST R 167565)
- Pack with 3 tablets 100 IR and 28 tablets 300 IR
- Pack with 3 tablets 100 IR and 7 tablets 300 IR
Oralair Continuation Treatment sublingual tablets 300 IR (AUST R 167566)
- Pack with 30 tablets
- Pack with 90 tablets
- Pack with 100 tablets
NAME AND ADDRESS OF THE SPONSOR AND DISTRIBUTOR:
Sponsor
Stallergenes Australia Pty Ltd
Suite 2408,
4 Daydream Street,
Warriewood,
New South Wales 2102
Ph: 1800 824 166
Distributed in Australia by:
EBOS Group Pty Ltd
Unit 2A Clayton Business Park
1508 Centre Rd, CLAYTON VICTORIA 3168
Distributed in New Zealand
EBOS Healthcare 14-18 Lovell Court Rosedale, Auckland
New Zealand
Ph: 0800 733 633
Fax : 0800 262 262
Date Prepared: 3 October 2013 Amended 13 Apr 2016